Secondary tumor of the kidney originating from ovarian carcinoma is an extremely rare phenomenon; this raises the theory of renal–ovarian axis. The poor understanding of this metastatic spread results in missed or delayed diagnosis of the secondary renal masses and delayed treatment of the ovarian metastasis. We describe here a case of renal metastasis diagnosed in a 60-year-old female within three years after a complete remission from a second incidence five years after the treatment of a stage IIIb high-grade serous ovarian cancer. An exhaustive review of published English reports of renal metastasis from ovarian cancer was undertaken to elucidate the concept of the renal-ovarian axis.
Advanced high-grade serous ovarian carcinoma is characterized by an 85% response rate to initial treatment. Of these,75% have a recurrence, which is typically incurable [1]. Usually, relapses from epithelial ovarian carcinoma are diagnosed within the first two years and over half of deaths occur within five years of diagnosis [2]. Secondary tumors of the kidney associated with ovarian carcinoma are extremely rare because ovarian carcinoma usually spreads along the peritoneum and throughout the pelvic and abdominal cavities [3]. We herein describe a case of renal metastasis of a high-grade serous ovarian carcinoma 8 years after the initial ovarian carcinoma was diagnosed.
In September 2008, a 60 year-old female was treated for a right ovarian mass (Fig. 1). She had a bilateral salpingo-oophorectomy, simple hysterectomy, appendicectomy, a total omentectomy with pelvic and para-aortic lymph node dissection. Histological examination of the ovarian mass revealed a high-grade serous carcinoma (Fig. 2) with nuclear expression of Wilms tumor-1 (WT1) (Fig. 3). A microscopic invasion of the serous of the appendix, and a peritoneal parietal cacinomatosis measuring less than 1.5 cm were also observed with no lymph node involvement. The patient was staged according to the International Federation of Gynecology and Obstetrics (FIGO) IIIB and received six cycles of adjuvant chemotherapy with Paclitaxel and Cisplatin.

After a complete response within five years, the patient presented with re-elevated CA125 levels (790U/mL) and a solitary mass of the left abdominal area measuring 70×50×25 mm was detected via computed tomography scan. The patient underwent a secondary complete cytoreductive surgery of the parietal mass, followed by six cycles of adjuvant chemotherapy with Paclitaxel and Cisplatin. These treatments resulted in a complete remission.
Follow-up tests showed no indication of relapse until April 2016, when the patient presented with upper right abdominal pain radiating to the back, asthenia, anorexia and weight loss. The computed tomography scan showed a heterogeneous and irregular lesion at the mid and lower pole of the right kidney measuring 100.9×178.8 mm with hydronephrosis and multiple liver and lung metastasis (Fig. 4).

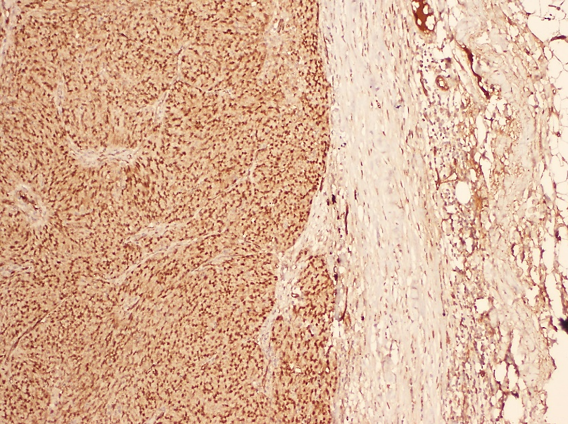
A tomography-guided percutaneous biopsy of the right renal mass was performed and the specimen showed a poorly differentiated proliferation with similar morphology to that of the ovarian tumor (Fig. 5) and also a nuclear expression of Wilms tumor-1 (WT1) in the immunohistochemical staining. These data support the hypothesis of metastatic origin of the renal mass from the previously treated ovarian mass (Fig. 6). The patient received 6 cycles of Paclitaxel and Cisplatin which resulted in a progression of the peritoneal and hepatic disease and the death of the patient.

Metastasis to the kidney is known to be a rare occurrence and data collected from autopsies suggest an incidence ranging from 2.36% to 12.6% [4, 6]. In the recent study Zhou et al. of 151 cases of secondary renal tumors, the most common primary tumor were lung (43.7%), colorectal (10.6%), head and neck (6%), breast (5.3%), soft tissue (5.3%) and thyroid (5.3%) and gynaecological primary tumors represented only 4.6% though metastatic ovarian cancer was not specifically described in their study [7].
To our knowledge, the first case of renal metastasis from an ovarian cancer was reported by Mandeville in 1949, who described a Dysgerminoma of the ovary in a 4-years-old girl with metastases clinically simulating Wilms' tumor and adrenal neuroblastoma [8].
From the nine cases discussed in our review, papillary cystadenocarcinoma constitutes three cases, making it the most common type in this sample [9, 11], high grade serous carcinoma made up two cases [12, 13], and the remaining case described clear cell carcinoma [14] Of these, Dysgerminoma occurred in two cases [8, 15] in addition to one case of ovarian granulosa cell tumor [16]. Until 2018, our patient represents the sixth reported case of renal metastasis from a serous ovarian carcinoma.
Most of the reported renal metastases, regardless the primary tumor, were typically solitary (77.5%) [7]. However, our patient presented with renal, liver and lung metastasis, consistent with three other reported cases [8, 9, 14].
| Authors | Age (Years) | symptoms | Primary/secondary site Solitary/polymetastatic | Synchronous / Metachronous | Cell type | TTT | Disease status |
|---|---|---|---|---|---|---|---|
| Mandeville [8] 1949 | 4 | Post mortem (Rupture of the left ovary) | Left ovary/left kidney Polymetastatic (liver, adrenal, pancreas, lymph node, and bone marrow involvement) | S | Dysgerminoma | - | death |
| Friedman [9] 1984 | 39 | Renal failure | Left ovary/left kidney Polymetastatic (distant LN) | M -9 years | Papillary cystadenocarcinoma | Total Nephrectomy + CT | Alive > 7 months |
| Cortes [12] 1986 | 49 | Abdominal swelling | Left ovary/left kidney Solitary renal metastasis | S | Serous cystadenocarcinoma | Total Nephrectomy + CT | Death 10 weeks |
| Gavallos [13] 2003 | 62 | Incidental screening | Left ovary/left kidney Solitary renal metastasis | M - 5 years | Serous Cystadenocarcinoma | Total Nephrectomy + CT | Alive > 6 months |
| Thyagarajan [10] 2008 | 70 | Abdominal pain | Left ovary/left kidney Solitary renal metastasis | M - 2 years | Papillary cystadenocarcinoma | Total Nephrectomy + CT | Alive |
| Wynter [11] 2012 | 38 | Incidental screening | Right ovary/bilateral solitary renal metastasis | M -3 years | Papillary cystadenocarcinoma | Partial Nephrectomy + CT | Alive > 3 years |
| Priyadarshi [15] 2013 | 24 | Lump Anemia Pain+ swelling of the left flank | Left ovary/left kidney Solitary renal metastasis | M - 1 year | Dysgerminoma | CT | Undergoing CT |
| Burns EM [16] 2013 | 45 | Flank pain | Left ovary/left kidney Solitary renal metastasis | M - 7 years | Granulosa | Total Nephrectomy + CT | Alive |
| Lohmann [14] 2016 | 62 | Abdominal pain Anorexia Weight loss Dyspnoe | Left ovary/left kidney Polymetastatic (Mediastinal LN+ Abdominal LN) | M - 4 years | Clear cell ovarian carcinoma | - | Death 3 weeks |
| Present case 2018 | 60 | Right flank and abdominal pain Asthenia Anorexia Weight loss | Right ovary/Right kidney Polymetastatic (liver+ lung) | M- 8 years | High grade serous carcinoma | CT | Death |
The majority of the published cases reported an ipsilateral left-sided ovarian carcinoma and renal metastases (Table 1), which support the theory of renal–ovarian axis as suggested by Gavallos et al. with the gonadal vein acting as a route for the spread of tumor between the left kidney and left ovary [13]. In our case, the primary ovarian tumor and the renal metastases occurred on the right side, which was only reported by Wynter et al. in 2012. This work described bilateral renal masses with a right-sided ovarian tumor and suggested more typical hematogenous pattern spread [11].
The diagnoses of renal masses in patients with a prior history of cancer presents a challenge given that radiological diagnostics alone are unable to distinguish between primary and secondary renal tumor, especially in the case of cystic lesions [17]. As renal metastasis from ovarian cancer remains rare, patients in most of the described cases underwent partial or radical nephrectomy (Table 1). For example, in many instances of ovarian tumors, WT1 is characteristic for the subtype of serous ovarian carcinoma and this marker, among others, can be used to diagnose metastasis of ovarian origin [18]. Therefore, we highly recommend renal biopsy with adequate immunohistochemical markers; these simple tests could significantly impact the management of patients both in the pharmacological approaches to controlling and eliminating the disease and by increasing the possibility of avoiding major surgery [11].
In our focal study, percutaneous biopsy was performed after we suggested the possibility of renal metastasis due to the patient’s history of relapse and polymetastatic disease. Microscopic similarity between the primary ovarian tumor and the renal mass with WT1 expression in the renal specimens helped to exclude the renal tumor as a primary cancer.
The management of metastatic malignancy involving the kidney typically uses chemotherapy based on and according to the histology of the primary tumor [17]. However, in the study of Zhou et al., curative surgery was performed in 44.7% of patients with oligometastatic disease involving the kidney and 21.3% of cases involved surgery for palliative purposes [7]. Due to rarity of renal metastasis of ovarian cancer and the poor prognosis of recurrent ovarian disease, treatment varies; multimodal therapy including surgery and/or chemotherapy are typically in most of the reported cases (Table 1). In our focal case, the patient presented with a polymetastatic recurrence which was managed exclusively with chemotherapy.
Renal metastasis from ovarian cancer is a rare and poses a perplexing problem of diagnosis; our work suggests that biopsy of renal mass after ovarian cancer should be performed to avoid nephrectomy, especially in cases of polymetastatic disease. Due to lack of data, there is little consensus as to optimal management of these tumor recurrences which are still treated according the existing guidelines of ovarian metastatic disease.
- Horowitz N, Miller A, Rungruang B, Richard S, Rodriguez N, Bookman M, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33:937-43 pubmed publisher
- Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13:125-9 pubmed
- Abrams H, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74-85 pubmed
- Klinger M. Secondary tumors of the genito-urinary tract. J Urol. 1951;65:144-53 pubmed
- Bracken R, Chica G, Johnson D, Luna M. Secondary renal neoplasms: an autopsy study. South Med J. 1979;72:806-7 pubmed
- MANDEVILLE F, SAHYOUN P, SUTTON L. Dysgerminoma of the ovary in a 4-year-old girl with metastases clinically simulating Wilms' tumor and adrenal neuroblastoma. J Pediatr. 1949;34:70-5 pubmed
- Friedman M, Browde S, Rabin S, Murray J, Nissenbaum M. Late metastases of ovarian carcinoma. A case report. S Afr Med J. 1984;65:178-9 pubmed
- Cortes J, Uriz A, Terrasa J, Rifa J, Rossello J, Llompart M. Ovarian cancer metastatic to the kidney: case report. Eur J Gynaecol Oncol. 1986;7:206-9 pubmed
- Gavallos G, Tawfik O, Herrell D, Langenstroer P. Renal-ovarian axis: a case report and review. Urology. 2003;62:749 pubmed
- . Lohmann S, Keller AK. A Case Report: Metastasis of Clear Cell Ovarian Cancer in Morrison’s Pouch as Differential Diagnosis to Exophytic Kidney Tumor. Open J Urol. 2016;6(11):173.
- . Priyadarshi V, Sarkar K, Singh JP, Loonia R, Chakrabarty D, Pal DK. Ovarian Dysgerminoma with Renal Metastasis: An Uncommon Phenomenon. UroToday Int J. 2013 June;6(3):art 31.